Latest Research News
-
7
Development of Stretchable and Printable Free-Form Lithium-Ion Batteries
- Realization of stretchable, adhesive, and mechanically deformable batteries that effectively transfer ions - Every component was designed to be stretchable to enable printing on clothing and use in wearable devices A Korean research team has developed a soft, mechanically deformable, and stretchable lithium battery which can be used in the development of wearable devices, and examined the battery’s feasibility by printing them on clothing surfaces. The research team, led by Dr. Jeong Gon Son from the Soft Hybrid Materials Research Center at the Korea Institute of Science and Technology (KIST; President: Seok-Jin Yoon), announced that they had developed a lithium battery wherein all of the materials, including the anode, cathode, current collector, electrolytes, and encapsulant, are stretchable and printable. The lithium battery developed by the team possesses high capacity and free-form characteristics suitable for mechanical deformation. Owing to the rapidly increasing demand for high-performance wearable devices such as smart bands, implantable electronic devices such as pace-makers, and soft wearable devices for use in the realistic metaverse, the development of a battery that is soft and stretchable like the human skin and organs has been attracting interest. The hard inorganic electrode of a conventional battery comprises the majority of the battery’s volume, making it difficult to stretch. Other components, such as the separator and the current collector for drawing and transferring charges, must also be stretchable, and the liquid electrolyte leakage issue must also be resolved. To enhance stretchability, the research team avoided using materials as had been done in other studies which were unnecessary for energy storage, such as rubber. Then, a new soft and stretchable organic gel material was developed and applied based on the existing binder material. This material firmly holds the active electrode materials in place and facilitates the transfer of ions. In addition, a conductive ink was fabricated using a material with excellent stretchability and gas barrier properties to serve as as a current collector material that transfers electrons and an encapsulant which can function stably even at a high voltage and in various deformed states without swelling due to electrolyte absorption. The battery developed by the team is also able to incorporate existing lithium-ion battery materials, as they exhibit excellent energy storage density (~2.8 mWh/cm2) of a level similar to that of commercially available hard lithium-ion batteries at a driving voltage of 3.3 V or higher. All of the constituent components of the team's stretchable lithium-ion battery possess the mechanical stability to maintain their performance even after repeated pulling of the battery 1,000 or more times, a high stretchability of 50% or above, and long-term stability in air. Moreover, the research team directly printed the electrode and current collector materials which they had developed on either side of an arm warmer made of spandex and applied a stretchable encapsulant to the material, demonstrating the ability to print a stretchable high-voltage organic battery directly on clothing. Using the resulting battery, the research team was able to continuously power a smart watch even when it was being put on, taken off, or stretched. Dr. Son at KIST stated that his team has developed a stretchable lithium-ion battery technology which provides both structural freedom as a result of the battery’s free-form configuration allowing for it to be printed on materials such as fabrics, and material freedom due to being able to use existing lithium-ion battery materials, in addition to stretch stability which allows for high energy density and mechanical deformation. He also stated that the stretchable energy storage system developed by his team is expected to be applicable to the development of various wearable or body-attachable devices. This study was supported by the Mid-Career Research Program of the National Research Foundation of Korea, and the KIST Institutional Program and K-Lab Program funded by the Ministry of Science and ICT (Minister: Hye-Sook Lim). The research results were published in ACS Nano (IF: 15.881). [1] Graphic image of the research [2] Schematic illustration of the assembled cell of the fully stretchable lithium-ion battery based on PCOG/active materials, SCCs, stretchable PCOG separator, and stretchable encapsulant printed on stretch fabric. [3] (a) Schematic illustration of the stretchable battery printed on stretch fabric consisting of printable stretchable electrodes, SCCs, encapsulant, and fabric as a stretchable separator. (b) Scanning electron microscope cross-sectional image of the stretchable battery printed on the stretch fabric. (c) Capacity change as a function of strain. (d) Change in the voltage and current of stretchable battery printed on the stretch arm sleeve under various angled deformations at the elbow. (e) Photographic images of a continuously operated smart watch connected with the stretchable lithium-ion battery printed our institute name on the stretch fabric before and after wearing and stretching
- 6
- WriterDr. Son, Jeong Gon
- 작성일2022.03.25
- Views1603
-
5
Another Step Taken Toward Commercialization of High-Safety All-Solid-State Lithium-Ion Batteries
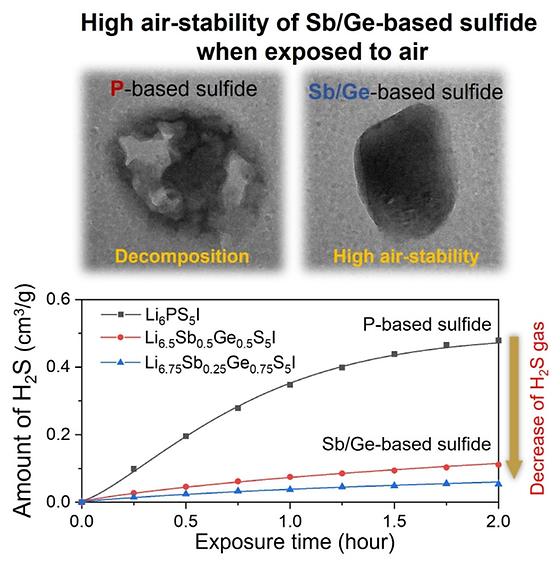
- Solid electrolytes with high ionic conductivity comparable to that of liquid electrolytes have been developed - Exhibits a 70% reduction in toxic hydrogen sulfide gas generation compared to other sulfide-based electrolytes when exposed to air Owing to the rapid growth of the electric vehicle and energy storage system (ESS) markets, the demand for lithium-ion batteries has been swiftly increasing. Conventional lithium-ion batteries use flammable liquid electrolytes and there have been continuous reports recently about such electrolytes causing accidents such as fires and explosions, raising concerns about their safety. Consequently, all-solid-state lithium-ion batteries using non-flammable solid electrolytes have been receiving significant attention as a next-generation secondary battery which can resolve these safety concerns. However, solid electrolytes have generally exhibited a low ionic conductivity in comparison to liquid electrolytes. The research team, led by Dr. Seungho Yu from the Energy Storage Research Center at the Korea Institute of Science and Technology (KIST, President: Seok Jin Yoon), recently developed a solid electrolyte with a high ionic conductivity, comparable to that of liquid electrolytes, by optimizing the material properties and synthesis process for sulfide solid electrolytes. Various candidate materials for solid electrolytes with a high ionic conductivity have been reported successively, and sulfide solid electrolytes exhibit a relatively high ionic conductivity, leading researchers to attempt to improve the material properties and synthesis process for sulfide electrolytes. However, sulfide solid electrolytes react with moisture when exposed to air, generating toxic hydrogen sulfide gas, which is a major concern. Therefore, further studies to resolve this issue were necessary. Dr. Yu’s team successfully developed a solid electrolyte with a high ionic conductivity of 16.1 mS/cm, by introducing antimony and germanium and inserting additional lithium into the argyrodite sulfide solid electrolytes. The ionic conductivity of these solid electrolytes is comparable to that of commercial liquid electrolytes (~10 mS/cm) and exceeds the maximum-level ionic conductivity of the previously developed argyrodite sulfide solid electrolytes (14.8 mS/cm). The research team then assembled a solid-state battery using the argyrodite sulfide solid electrolytes and obtained a similar initial discharge capacity to that of a liquid electrolyte Li-ion battery. These results are promising for the subsequent development of all-solid-state lithium batteries with a high energy capacity and long lifecycle through optimization of the fabrication process. While existing sulfide solid electrolytes react with moisture when exposed to air, leading to the evolution of toxic hydrogen sulfide gas, this study resulted in the successful reduction of hydrogen sulfide gas evolution by more than 70% through the introduction of antimony to minimize the reaction with moisture. Dr. Yu at KIST stated that “the solid electrolytes developed in this study exhibit a high ionic conductivity comparable to that of liquid electrolytes, and significantly improved air-stability, which is expected to accelerate the commercialization of all-solid-state lithium batteries.” This study was supported by the KIST Institutional Program and the Technology Development Program to Solve Climate Change of the National Research Foundation of Korea funded by the Ministry of Science and ICT of Korea (Minister: Hye-Sook Lim); by the Lithium-based Next-Generation Secondary Battery Performance Advancement and Manufacturing Technology Development Program, and the Automobile Industry Core Technology Development Program funded by the Ministry of Trade, Industry and Energy of Korea (Minister: Sung Wook Moon). The research findings were published in the latest issue of the international journal ACS Energy Letters (IF: 23.101, top 3.302% in the JCR field). [1] Solid electrolytes with high ionic conductivity Schematic illustration of the synthesis process, Li-ion migration path, and Li-ion conductivity of Li6.5Sb0.5Ge0.5S5I. [2] Solid electrolytes with high air-stability Images of P and Sb/Ge based sulfides after exposure to air and their amount of H2S generation. [3]Corresponding Author(Dr. Yu, Seungho)
- 4
- WriterDr. Yu, Seungho
- 작성일2022.03.25
- Views1458
-
3
Producing ethylene from food waste without greenhouse gas emissions
Technology developed to remove and overcome toxic hydrogen sulfide from the production process. Great help expected for domestic chemical companies to achieve carbon neutrality The Korea Institute of Science and Technology (KIST, President Dr. Yoon, Seok-Jin) announced that a research team led by Dr. Jeong-Myeong Ha of the Clean Energy Research Center developed a process technology and catalyst for removing hydrogen sulfide, a toxic substance, during the process of ethylene production from methane in biogas. <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"="">Biogas, which is produced by microorganisms present in food waste, livestock manure, and sewage sludge, consists mainly of methane that can be used for low-cost energy including power generation, heating, and addition to town gas. Methane can acquire a large added value if converted into ethylene, a basic raw material for industries, through chemical reactions. Ethylene is a typical non-petroleum product that can reduce greenhouse gases. <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"="">The research team developed a process technology in 2021 that produces ethylene from biogas with the help of catalysts. In addition to methane, which is fairly useful in general, biogas contains a significant amount of toxic hydrogen sulfide, which is difficult to remove and interferes with the catalytic reaction in ethylene production. The developed technology facilitates ethylene production by oxidizing and removing hydrogen sulfide during the production process. <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"="">The researchers then developed a catalyst to improve reaction activity of ethylene production from biogas and methane. This catalyst is highly resistant to hydrogen sulfide, thus not requiring hydrogen sulfide removal from biogas, while the energy consumption can be reduced by lowering the operating temperature from 800oC to 700oC due to improved reaction activity. Through such reaction, it is possible to directly produce ethylene from biogas that contains hydrogen sulfide. Dr. Jeong-Myeong Ha stated, "Biogas is already produced in large quantities in Korea, and if biogas is used as a raw material for the chemical industry rather than just for heating, biogas producers who struggle to achieve carbon neutrality will have a larger market and be able to provide new raw materials without greenhouse gas emissions." He also mentioned, "This technology will draw attention from related companies as it can utilize not only biogas but also methane generated from various wastes such as plastics." <span style="background-color: rgb(255, 255, 255); color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;"=""> Image <img src="/Data/editor/2022030814410843_0.png" title="2022030814410843_0.png" alt="" style="color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;"=""> <span style="background-color: rgb(255, 255, 255); color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;"=""> <span style="background-color: rgb(255, 255, 255); color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;"="">
- 2
- WriterDr. Ha, Jeong-Myeong
- 작성일2022.03.04
- Views1334
-
1
Improved fuel cell performance using semiconductor manufacturing technology
Development of metal nanoparticle synthesis methods for eco-friendly mass production using sputtering application, a metal deposition technology. Applicable to high-performance hydrogen fuel cell catalysts A research team in Korea has synthesized metal nanoparticles that can drastically improve the performance of hydrogen fuel cell catalysts by using the semiconductor manufacturing technology. The Korea Institute of Science and Technology (KIST, President Seok Jin Yoon) announced that the research team led by Dr. Sung Jong Yoo of the Hydrogen Fuel Cell Research Center has succeeded in synthesizing nanoparticles by a physical method rather than the existing chemical reactions by using the sputtering technology, which is a thin metal film deposition technology used in semiconductor manufacturing. <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"="">Metal nanoparticles have been studied in various fields over the past few decades. Recently, metal nanoparticles have been attracting attention as a critical catalyst for hydrogen fuel cells and water electrolysis systems to produce hydrogen. Metal nanoparticles are mainly prepared through complex chemical reactions. In addition, they are prepared using organic substances harmful to the environment and humans. Therefore, additional costs are inevitably incurred for their treatment, and the synthesis conditions are challenging. Therefore, a new nanoparticle synthesis method that can overcome the shortcomings of the existing chemical synthesis is required to establish the hydrogen energy regime. <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"=""> <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"="">The sputtering process applied by the KIST research team is a technology that coats a thin metal film during the semiconductor manufacturing process. In this process, plasma is used to cut large metals into nanoparticles, which are then deposited on a substrate to form a thin film. The research team prepared nanoparticles using 'glucose', a special substrate that prevented the transformation of the metal nanoparticles to a thin film by using plasma during the process. The synthesis method used the principle of physical vapor deposition using plasma rather than chemical reactions. Therefore, metal nanoparticles could be synthesized using this simple method, overcoming the limitations of the existing chemical synthesis methods. <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"=""> <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"="">The development of new catalysts has been hindered because the existing chemical synthesis methods limited the types of metals that could be used as nanoparticles. In addition, the synthesis conditions must be changed depending on the type of metal. However, it has become possible to synthesize nanoparticles of more diverse metals through the developed synthesis method. In addition, if this technology is simultaneously applied to two or more metals, alloy nanoparticles of various compositions can be synthesized. This would lead to the development of high-performance nanoparticle catalysts based on alloys of various compositions. <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"=""> <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"="">The KIST research team synthesized a platinum-cobalt-vanadium alloy nanoparticle catalyst using this technology and applied for the oxygen reduction reaction in hydrogen fuel cell electrodes. As a result, the catalyst activity was 7 and 3 times higher than those of platinum and platinum-cobalt alloy catalysts that are commercially used as catalysts for hydrogen fuel cells, respectively. Furthermore, the researchers investigated the effect of the newly added vanadium on other metals in the nanoparticles. They found that vanadium improved the catalyst performance by optimizing the platinum?oxygen bonding energy through computer simulation. <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"=""> Dr. Sung Jong Yoo of KIST commented, “Through this research, we have developed a synthesis method based on a novel concept, which can be applied to research focused on metal nanoparticles toward the development of water electrolysis systems, solar cells, petrochemicals.”. He added, “We will strive to establish a complete hydrogen economy and develop carbon-neutral technology by applying alloy nanoparticles with new structures, which has been difficult to implement, to development eco-friendly energy technologies including hydrogen fuel cells.” <span style="background-color: rgb(255, 255, 255); color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;"=""> Image <img src="/Data/editor/2022030814320838_0.jpg" title="2022030814320838_0.jpg" alt="" style="color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;"=""> <span style="background-color: rgb(255, 255, 255); color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;"=""> ILLUSTRATION OF THE STEP-BY-STEP SYNTHESIS PROCESS FOR THE PREPARATION OF TERNARY NANOPARTICLE CATALYSTS AND ELECTRON STRUCTURE REARRANGEMENT BY ELECTRON TRANSFER BETWEEN METAL ATOMS.
- 0
- WriterDr. Sung Jong Yoo
- 작성일2022.02.28
- Views1383
-
-1

Low-temperature DeNOx catalyst for reducing ultrafine particle emission
7 times increased durability compared to conventional commercial catalysts. Empirical research conducted at an industrial field to check commercialization (Kumho Petrochemical Cogeneration Power Plant) <span style="background-color: rgb(255, 255, 255); color: rgb(51, 51, 51); font-family: 나눔고딕코딩, NanumGothicCoding, sans-serif; font-size: 14pt;" open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;"="">Recently, there has been growing demand for DeNOx catalysts that can treat nitrogen oxides (NOx) at low temperatures, to increase energy efficiency when processing flue gas in industrial combustion facilities. NOx are emitted during the combustion of fossil fuels and are the leading cause of ultrafine particles (UFPs) formed via chemical reactions in the atmosphere. <span style="background-color: rgb(255, 255, 255); color: rgb(51, 51, 51); font-family: 나눔고딕코딩, NanumGothicCoding, sans-serif; font-size: 14pt;" open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;"=""> <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"="">However, existing catalysts have a problem of reduced durability due to the poisoning of the catalyst’s active sites because of the formation of ammonium sulfate, when sulfur in flue gas reacts with reducing agent ammonia at a low temperature (<250°C). To address this, studies have attempted to weaken the oxidation ability of sulfur oxide on the catalyst surface or delay the poisoning by limiting the reactivity of sulfur compounds; however, these solutions cannot increase the durability against sulfur. <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"=""> <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"="">At the Extreme Materials Research Center, part of the Korea Institute of Science and Technology(KIST), a research team of Dr. Kwon, Dong Wook and Dr. Ha, Heon Phil announced the development of a high-durability low-temperature catalyst material for selective catalytic reduction (SCR); it can reduce NOx into water and nitrogen, which are harmless to the environment and the human body. <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"=""> <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"="">The team successfully developed a composite vanadium oxide-based catalyst material that significantly limited the formation of poisonous ammonium sulfate by suppressing the adsorption reaction between the active sites and sulfur dioxide. A catalyst interface engineering technique was used in which molybdenum and antimony oxide were added to the vanadium-based catalyst. <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"=""> <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"="">The developed vanadium oxide-based composite catalyst material has significantly increased catalytic life when exposed to sulfur dioxide at 220°C, with the time to reach 85% of the initial performance delayed by about seven times compared to that in the conventional catalyst. The developed catalyst is also energetically efficient due to increased low-temperature activity, which significantly lowers the burden of NOx treatment without reheating the exhaust gas. As a result, it is possible to reduce air pollutant treatment costs if the developed catalyst is applied to industrial sites in the future. <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"=""> <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"="">After completing the laboratory-scale reactor experiment, the team installed a pilot demonstration facility at the Kumho Petrochemical’s Yeosu 2nd Energy Cogeneration Power Plant to test using actual flue gas. The KIST-Kumho Petrochemical team aims to establish plant facilities by 2022 after deriving an optimal operation plan by evaluating and verifying the driving variables of the demonstration facility for about ten months. <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"=""> <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"="">Ko, Young Hoon, the head of R&BD center of Kumho Petrochemical (Vice-President), mentioned, “Reducing NOx, which accounts for most of the harmful substances in the exhaust gas of our Cogeneration Power Plant, is a critical issue for Kumho Petrochemical’s ESG management.” Then, he added, “We are successfully conducting empirical research by installing pilot equipment for power plants to secure preemptive reduction technology above the level of advanced countries, and we plan to conduct scale-up test of the technology in order to transform it to a high-durability low-temperature SCR catalytic commercial technology.” <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"=""> <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"="">Image <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"=""> <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); text-align: center;" open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"=""> <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"=""> <p style="text-align: center; box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51);" open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"="" align="center">SCR PILOT DENOX REACTOR THROUGH ON-SITE EXHAUST GAS INJECTION.
- -2
- WriterDr. Kwon, Dong Wook and Dr. Ha, Heon Phil
- 작성일2022.01.15
- Views1388
-
-3
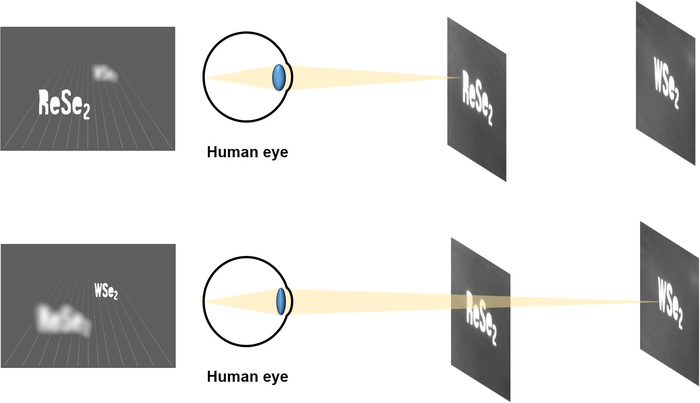
3D Digital Holograms on Smartphones?
Realized 3D digital holograms by developing a polarization image sensor with no additional polarization filters. Miniaturization of the entire holographic camera sensor module is possible with follow-up research 3D holograms, previously seen only in science fiction movies, may soon make their way into our daily lives. Until now, 3D holograms based on phase shifting holography method could be captured using a large, specialized camera with a polarizing filter. However, a Korean research group has just developed technology that can acquire holograms on mobile devices, such as smartphones. <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);="" line-height:="" 1.5;"="">The Korea Institute of Science and Technology (KIST, Director Seok-jin Yoon) recently announced that a research team led by Dr. Min-Chul Park and Dr. Do Kyung Hwang of the Center for Opto-Electronic Materials and Devices, in collaboration with a research team led by Prof. Seongil Im of the Department of Physics at Yonsei University, was successful in developing a photodiode that detects the polarization of light in the near-infrared region without additional polarization filters and thus, the realization of a miniaturized holographic image sensor for 3D digital holograms, using the 2D semiconductor materials: rhenium diselenide and tungsten diselenide. <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);="" line-height:="" 1.5;"=""> <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);="" line-height:="" 1.5;"="">Photodiodes, which convert light into current signals, are essential components within the pixels of image sensors in digital and smartphone cameras. Introducing the ability to sense the polarization of light to the image sensor of an ordinary camera provides a variety of new information, enabling the storage of 3D holograms. Previous polarization-sensing cameras have an additional polarization filter, several hundred micrometers in size, attached to an ultra-small optical diode image sensor, less than a micrometer in size. Thus, they could not be implemented into portable electronic devices because of their inability to be integrated and miniaturized. <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);="" line-height:="" 1.5;"=""> <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);="" line-height:="" 1.5;"="">The research group developed a photodiode by stacking an n-type semiconductor, rhenium diselenide, which exhibits a difference in light absorption dependent on the linear polarization angle of light in the near-infrared (980 nm) region, and a p-type semiconductor, tungsten diselenide, which exhibits no difference in photo-response dependent on polarization, but enables superior performance. The device is excellent in the photodetection of various wavelengths from ultraviolet to near-infrared, even capable of selectively detecting the polarization characteristics of light in the near-infrared region. The research group utilized the device to create a digital holographic image sensor that records polarization characteristics to successfully capture holograms. <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);="" line-height:="" 1.5;"=""> Dr. Hwang of KIST said, "Research on the downsizing and integration of individual elements is required to ultimately miniaturize holographic systems. The results of our research will lay the foundation for the future development of miniaturized holographic camera sensor modules." In addition, Dr. Park remarked, "The new sensor can further detect near-infrared light, as well as previously undetectable visible light, opening up new opportunities in various fields such as 3D night vision, self-driving, biotechnology, and near-infrared data acquisition for analyzing and restoring cultural assets." Image HOLOGRAM IMPLEMENTED WITH TWO-DIMENSIONAL SEMICONDUCTOR WSE2/RESE2, WHICH IS A POLARIZATION-SENSING PHOTODIODE, RESE2 ON THE FRONT AND WSE2 ON THE BACK ARE IMAGED IN THREE-DIMENSIONAL SPACE
- -4
- WriterDr. Min-Chul Park and Dr. Do Kyung Hwang
- 작성일2022.01.05
- Views2004
-
-5
Low concentrations of CO2→CO direct conversion technology
Flue gas level low-concentration carbon dioxide high-efficiency conversion made possible. Economically feasible electrochemical carbon dioxide conversion achieved A Korean research team has developed a technology that can produce carbon monoxide (CO), which has various applications in industry, by direct conversion of flue gas level low-concentration carbon dioxide (CO2). The Korean Institute of Science and Technology (KIST, President Seok-jin Yoon) announced that the research team of Dr. Da Hye Won and Dr. Ung Lee at Clean Energy Research Center and Professor Yun Jeong Hwang at Seoul National University (President Se-jung Oh) has developed a catalyst and a operating process that can produce CO with high efficiency using flue gas level low-concentration CO2. <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"="">CO2 conversion to valuable chemicals is one of the most promising strategies for mitigating the global climate crisis and developing new processes for chemical production.However, these technologies require supply of high-concentration CO2 gas. This is because CO2 is chemically very stable, which makes it difficult to convert CO2 to other chemicals, and a high-concentration CO2 supply is needed to increase the reaction rate and the efficiency. Actual flue gas from industrial plants typically contains 10% CO2 along with other emissions such as nitrogen, oxygen, and nitrogen oxide; however, until now, it has not been possible to achieve enough efficiency from this low concentration of CO2. <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"="">As a catalyst for electrochemical conversion of CO2 to CO, Ag is mainly used due to its high CO productivity. Commercial Ag nanopacticles produce 95% CO when high-concentration CO2 (99.99%) is used, while it produces 40% CO and 60% hydrogen when low-concentration (10%) CO2 is used. <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"="">The research team at KIST has developed a nickel single-atom catalyst that can inhibit hydrogen production and increase CO production efficiency. Transition metals such as iron and nickel could not be used as CO2 conversion catalysts due to their lower reactivities than those of noble metals; however, the recent finding that using transition metals in single-atom structure can achieve high CO productivity motivated the team to develop the new catalyst. The team also developed an optimized operating techniques for advanced CO2 conversion system that can directly convert gas-fed low-concentration CO2 by using both experimental and computational simulation methods. <p style="box-sizing: border-box; margin-top: 5px; margin-bottom: 15px; color: rgb(51, 51, 51); font-family: " open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;="" background-color:="" rgb(255,="" 255,="" 255);"="">The developed nickel single-atom catalyst can produce 93% CO with low-concentration (10%) CO2, and it is also economically feasible by using non-precious materials composed of nickel and carbon compared to noble Ag catalyst. Dr. Da Hye Won at KIST said, “The developed catalyst and the operating techniques can be widely applied in electro-chemical conversion systems utilizing low-concentration carbon dioxide. And we are also in the process of development of various technologies to use the flue gas directly without any additional conditioning process to achieve the economic feasibility of electro-chemical carbon dioxide conversion technology.” Image DEVELOPED EXTRINSIC OPERATING CONDITIONS CONTROLLING THE WATER TRANSFER FROM THE ANOLYTE TO THE CATALYST LAYER AND IMPROVED CO SELECTIVITY AT LOW CO2 CONCENTRATIONS IN THE MEA ELECTROLYZER. <span open="" sans",="" "helvetica="" neue",="" helvetica,="" arial,="" sans-serif;="" font-size:="" 14px;"="" style="background-color: rgb(255, 255, 255); color: rgb(51, 51, 51);">
- -6
- WriterDr. Da Hye Won and Dr. Ung Lee
- 작성일2021.12.08
- Views1508